A different way to treat
depression, anxiety, OCD & more.
depression, anxiety, OCD & more.
• • Accepting New Patients• •
There’s more to mental health treatment than medication.
Discover the TMS difference.

FDA-cleared, non-medication treatment is here: Introducing TMS
If you’re experiencing depression symptoms, unwanted side effects, or if you want to supplement your current treatments, you might consider TMS.
Transcranial Magnetic Stimulation (TMS) therapy is a non-invasive, non-drug, and FDA-cleared treatment with zero downtime that is safe and effective. TMS has fewer side effects than other treatments, and in some cases, there are no side effects at all. Plus, TMS is covered by insurance— like mental health treatments should be.
How TMS Works
FDA-Cleared.
Antidepressants have worked to treat depression for many people since the 1980s, but they don’t work for everyone. Instead, TMS works faster and with fewer side effects by using gentle magnetic pulses.
Non-Invasive.
TMS is non-invasive, non-sedating and well-tolerated. Patients can drive themselves to and from sessions at our private treatment centers and get back to their day right away, including work or school.
Drug-Free
Unlike medications, which affect the entire body, TMS therapy treats the brain directly. So, you don’t have to tolerate drug-related side effects, like gastrointestinal problems, trouble sleeping, or weight gain.
Therapy tailored specifically to you
TMS is highly-personalized to your unique anatomy. During the first appointment, your psychiatrist will create your individualized treatment plan by identifying the best magnet strength and coil position for you.
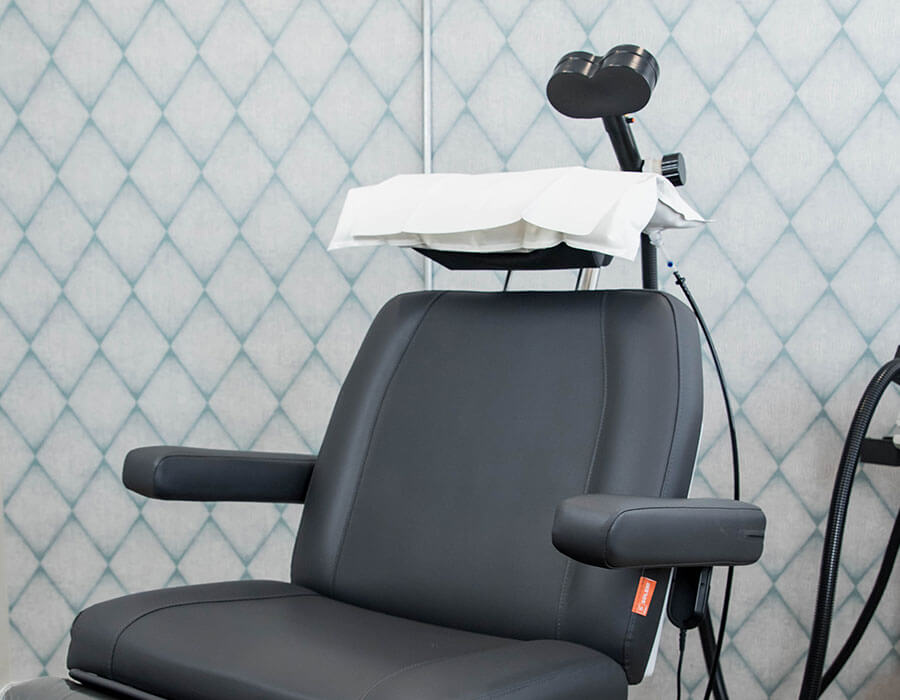
During each treatment session, you may relax, read, work, or watch television in our comfortable and private treatment rooms. A TMS magnet is positioned over your head to deliver gentle magnetic pulses to stimulate specific areas of the brain known to be under-active in depression.
Stimulating these areas improves the brain’s ability to regulate mood. So, TMS can be used as a stand-alone treatment or along with your existing medication.
Most of our patients say that the gentle pulses feel like taps. The sensation may take some getting used to, but isn’t painful. If you’re ever feeling any discomfort, your TMS technician can make adjustments.
Finally say goodbye to medication side effects
Searching for the right mental health treatment can be frustrating. No one should have to deal with lingering symptoms or unwanted side effects. Not to mention, symptoms can interfere with the comfort and effectiveness of talk therapy.
Many of our patients have had tried multiple different treatments before achieving full remission with TMS. Unlike medications which can take 6-8 weeks to exert their full effects, most of our patients experience improvement in 2 weeks with TMS therapy.
TMS doesn’t cause systemic side effects. Plus, TMS therapy is covered by most insurance, including Aetna, Anthem, Blue Cross Blue Shield, Humana, United Healthcare, and others.
TMS can help you feel better, faster
Some people with depression and other mental health conditions are able to achieve symptom remission with medication. However, antidepressant medications are not effective and tolerable for everyone. It may take up to 10 weeks after starting an antidepressant to notice improvement, and even then, symptom improvement only occurs in about one-third of patients.
Other patients prefer not to use medications. Because TMS treatment is non-invasive, non-systemic, and has fewer side effects than medications. Many people including those who are pregnant or breastfeeding choose TMS over other treatment options.


Experts agree there’s no debate that TMS works
With more than 10,000 treatments performed in clinical trials, there’s no debate that TMS works. So, what can TMS treat? TMS therapy is globally recognized for its profound effects on many mental health conditions including depression, OCD, migraine and cigarette cessation.
More research is published everyday and suggests TMS has the potential to expand its indications to include anxiety, memory loss, substance abuse treatment and more.
Get Started
1. Connect with our team
Get started with a brief chat, conveniently offered over the phone, and complete our intake questionnaire.
2. Schedule your first appointment
In-network appointments with our expert care team are available in days, not months
3. Meet your provider
Meet your expert care provider and get started with the best treatment for your unique health goals.

Our Locations

Pasadena











Questions? We’ve Got Answers.
-
Why choose TMS therapy?
For many years, the initial response to mental health treatment has been psychiatric medications and therapy. Although these conventional treatments can be effective, they don’t work for everyone and medications can take weeks or months to exert their full effects. If you’re experiencing symptoms, unwanted side effects, or if you want to supplement your current treatments, you might consider TMS.
Unlike other treatments, TMS is non-invasive (does not require surgery) and non-systemic (does not affect the entire body). TMS is covered by most insurance and has fewer side effects than other treatments, or in some cases, no side effects at all. TMS therapy is an FDA-cleared treatment that can be used alone or in conjunction with medication. TMS uses using gentle magnetic pulses to treat specific areas of the brain known to be underactive in conditions affecting mental health and cognition. Unlike medications, which affect the entire body, TMS therapy treats mental health at the source.
-
What is the effectiveness of TMS therapy?
TMS is a safe, highly effective and FDA-cleared treatment option for depression, OCD and cigarette cessation. Since 2009, over 70% of Neuro Wellness Spa patients have experienced symptom relief with TMS therapy. Although many mental health conditions are chronic, TMS helps patients achieve remission. Length of remission depends on many factors including:
- Diet
- Exercise
- Therapy
- Severity of illness
- Lifestyle
The goal of mental health treatment is to achieve full remission. Patients should not tolerate lingering symptoms or unwanted side effects. TMS is an effective treatment alternative to medication and therapy that helps patients feel all the way better.
-
What are the side effects of TMS therapy?
Transcranial magnetic stimulation has far fewer side effects than antidepressant medications. With more than 10,000 treatments performed in clinical trials, the most common side effect was temporary and mild scalp discomfort during active treatment. Unlike medications, which affect the entire body as they pass through the blood-brain barrier, TMS therapy treats the brain directly.
-
Does TMS only work with depression?
Since its FDA clearance in 2008 for depression and 2018 for OCD and cigarette cessation, TMS is also a promising treatment for a range of other conditions affecting mental health and cognition. Notably, TMS therapy is a safe and effective treatment for anxiety, cognitive decline, memory loss, and migraines. TMS therapy has also shown promise off-label for bipolar disorder, borderline personality disorder, peripartum depression, OCD, schizophrenia, and cigarette cessation.
-
What to expect during TMS treatment?
TMS is an outpatient procedure. Patients recline in a private treatment room and may relax, read, work, or watch television during treatment. A TMS magnet is positioned over the patient’s head to deliver gentle magnetic pulses to stimulate specific areas of the brain. Each treatment sessions lasts about 20 minutes, after which patients can get back to their days right away, including work or school.
Unlike electroconvulsive therapy or ECT, TMS therapy is non-invasive and non-sedating. TMS does not involve electrode implantation or the use of anesthesia. TMS treatment sessions take place in our private centers. Patients can drive themselves to and from treatment. There is no downtime, so patients can go about daily activities immediately following treatment. TMS treatment does not cause seizures nor affect cognitive function, it has fewer side effects than other treatments, and in some cases, no side effects at all.
-
When will I see results with TMS therapy?
Typically, TMS patients are treated 5 times per week for 6 weeks and then 2 times per week for 3 weeks. Each session lasts on average about 20 minutes but can be as short as three minutes with the theta burst protocol. Patients drive themselves to and from TMS and there is no interruption in daily activities. Most patients experience clinical improvement by the tenth session (two weeks)— not full remission but noticeable improvements in sleep, energy, motivation or lessened irritability and tearfulness.
-
How is TMS different from other mental health treatments?
Many of our patients have tried several treatments before finding success with TMS. TMS works differently than medications and talk therapy. Medications aren’t as customizable as TMS, and many patients experience a trial and error approach to choosing the best antidepressant. Medication trials can take months and oral medications are known to cause systemic side effects as they pass through the blood-brain barrier. Plus, certain antidepressants may not work for genetic reasons.
Symptoms of biological depression, especially when sub-optimally treated, can significantly interfere with the effectiveness of talk therapy.
In contrast, TMS treats mental health at the source. TMS targets the area of the brain that is believed to regulate mood using gentle magnetic pulses that are highly personalized to your unique anatomy. In doing so, it changes the way the neurons fire and function rather than just allowing the neurons to be bathed in more chemicals. TMS works faster than medications, with most patients noticing improvement in 2 weeks compared to 6-8 weeks with medications.
-
What do experts say about TMS therapy?
Extensive research and highly-effective treatment outcomes have greatly contributed to several notable publications about TMS:
- Mayo Clinic. “Transcranial magnetic stimulation (TMS) is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when other depression treatments haven’t been effective.” Read More.
- WebMD “TMS, in particular, is not only effective, but also very safe and well-tolerated by patients.” Read More.
- Journal of Clinical Psychology “Multiple randomized controlled trials and published literature have supported the safety and efficacy of rTMS antidepressant therapy.” Read More.
- Harvard Medical School “These (TMS therapies) are very effective options for a large percentage of people.”
- Ted Talk For over 25 years, magnets have been used to stimulate the brain to investigate how nerve cells are connected in the brain. Watch Sandro Krieg explain the potential of brain stimulation. Watch Now.
- Dr. Oz Dr. Oz investigates Transcranial Magnetic Stimulation (TMS), a groundbreaking therapy for depression that’s both noninvasive and FDA-approved. Could this work for you? Watch Part 1. Watch Part 2.
- American Psychological Association. “A treatment for adults with major depression who have failed to respond to at least one antidepressant.” Read More.
- Today “Helping people with depression when nothing else works.” Read More.
Contact us today to learn more about TMS or to schedule a consultation. Get started today.
-
Can I do TMS if I have dental fillings or piercings?
Yes! Only magnetic material interferes with TMS. Dental fillings and body piercings are rarely magnetic. If you have never been told you cannot have an MRI, you can do TMS.
-
If I get TMS therapy, can I stop taking medications?
If you’re currently taking medications that are well tolerated and effective, continuing current medications can help sustain your TMS response. Patients who are experiencing many side effects, however, sometimes elect to taper medications in collaboration with their psychiatrist.
-
What about insurance coverage for TMS therapy?
Transcranial magnetic stimulation therapy for depression is FDA-cleared and covered by most insurance including Aetna, Anthem, Blue Shield, Optum, MHN/Healthnet, Tricare, Cigna, Magellan and Beacon and more. Our phenomenal clinical team is always available to help prospective patients understand their coverage options. We know how important it is that those seeking help are able to get the treatment they need. Not to mention, at Neuro Wellness Spa, we handle all the paperwork.
-
Can TMS really cure depression?
TMS is a very powerful treatment, but it is not a cure. Depression is a chronic illness, one, like cancer, that can go into remission and never recur. However, it may recur. Continuing to take an antidepressant, if there is one tolerated with no side effects, is ideal as that provides greater support for maintaining remission after TMS. If you have a recurrence, you can return to TMS. Insurance usually covers TMS again if there has been 6 months of remission. Only about 15% of patients suffer a depressive recurrence within one year after completing TMS.
-
Don’t see your question here?
Let’s fix that. Give our expert clinical team a call or schedule a consultation.